Calcineurin Inhibitor: Unraveling the Mechanisms, Medical Applications, and Future Perspectives
Calcineurin inhibitors (CNIs) are a class of immunosuppressive drugs that have revolutionized the field of transplantation and the management of certain autoimmune diseases. These drugs work by inhibiting the activity of the enzyme calcineurin, a critical regulator of T-cell activation and the immune response. The discovery and development of calcineurin inhibitors have significantly improved the success rates of organ transplantation and provided new treatment options for patients with autoimmune conditions. This article explores the mechanisms of calcineurin inhibition, its medical applications, and the potential future perspectives in this field.
Mechanisms of Calcineurin Inhibition
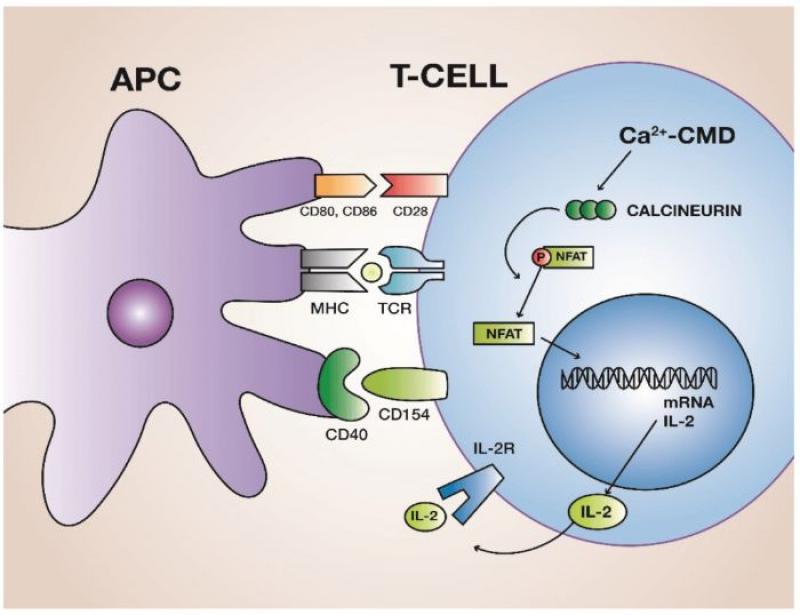
Calcineurin is a protein phosphatase that plays a crucial role in T-cell activation. When T-cells encounter foreign antigens, a signaling cascade is initiated, leading to the activation of calcineurin. Activated calcineurin dephosphorylates the nuclear factor of activated T-cells (NFAT), allowing NFAT to translocate into the nucleus. Once in the nucleus, NFAT collaborates with other transcription factors to induce the expression of cytokines, including interleukin-2 (IL-2), which promotes T-cell proliferation and activation.
Calcineurin inhibitors, such as cyclosporine and tacrolimus, act by binding to specific intracellular proteins called immunophilins. The drug-immunophilin complex then inhibits the phosphatase activity of calcineurin, preventing the dephosphorylation and nuclear translocation of NFAT. Consequently, the production of IL-2 and other pro-inflammatory cytokines is suppressed, leading to a dampened immune response.
Medical Applications of Calcineurin Inhibitors
1. Organ Transplantation
The most significant medical application of calcineurin inhibitors is in the field of organ transplantation. When a patient receives a transplanted organ, the body recognizes the graft as foreign and mounts an immune response to reject it. To prevent organ rejection, patients undergo immunosuppressive therapy, with calcineurin inhibitors playing a central role in the regimen.
Calcineurin inhibitors are typically used in combination with other immunosuppressive agents, such as corticosteroids and antimetabolites. This combination therapy aims to suppress the recipient's immune response sufficiently to prevent rejection while minimizing the risk of infection and other complications.
2. Autoimmune Diseases
Calcineurin inhibitors have also found applications in the treatment of certain autoimmune diseases. Conditions such as psoriasis, atopic dermatitis, and autoimmune uveitis have been shown to respond well to topical or systemic calcineurin inhibitor therapy.
In the case of psoriasis and atopic dermatitis, topical calcineurin inhibitors, such as pimecrolimus and tacrolimus, are used to reduce inflammation and manage symptoms. These drugs offer an alternative to corticosteroids, which may have long-term adverse effects on the skin with prolonged use.
3. Nephrology
In addition to their role in transplantation, calcineurin inhibitors have therapeutic applications in nephrology. For instance, tacrolimus is used to manage certain glomerular diseases, such as minimal change disease and membranous nephropathy. By suppressing the immune response, these drugs can reduce glomerular inflammation and proteinuria.
Challenges and Side Effects
While calcineurin inhibitors have significantly improved patient outcomes in transplantation and autoimmune disease management, they are not without challenges and side effects.
1. Nephrotoxicity
One of the main concerns with calcineurin inhibitor use is nephrotoxicity. These drugs can impair kidney function, leading to reduced glomerular filtration rate and potential long-term kidney damage. Regular monitoring of kidney function and dosage adjustments are essential to mitigate this risk.
2. Immunosuppression and Infections
Immunosuppressive therapy, including calcineurin inhibitors, increases the risk of infections. Patients on these drugs may be more susceptible to bacterial, viral, and fungal infections. Close monitoring for signs of infection and prompt intervention are crucial in managing this risk.
3. Drug Interactions
Calcineurin inhibitors are metabolized in the liver by the cytochrome P450 enzyme system. As a result, these drugs can interact with other medications metabolized by the same pathway, leading to altered drug levels and potential adverse effects.
Future Perspectives
The future of calcineurin inhibitors lies in the development of novel agents that offer improved efficacy and reduced toxicity profiles. Researchers continue to explore selective calcineurin inhibitors that target specific isoforms of the enzyme, aiming to minimize adverse effects while preserving immunosuppressive activity.
Additionally, drug delivery innovations, such as localized drug delivery and targeted nanoparticles, may enhance the therapeutic index of calcineurin inhibitors. These advancements could potentially reduce systemic exposure and improve drug bioavailability at the target site, further optimizing treatment outcomes.
Furthermore, ongoing research aims to better understand the complex mechanisms of calcineurin inhibition and its impact on the immune system. This deeper understanding may lead to more tailored and personalized treatment regimens, optimizing the balance between immunosuppression and preservation of protective immune responses.
Calcineurin inhibitors represent a significant milestone in the field of immunosuppressive therapy, offering new hope for patients undergoing organ transplantation and those with certain autoimmune diseases. By inhibiting calcineurin, these drugs effectively suppress the immune response, reducing the risk of organ rejection and managing inflammation in autoimmune conditions. Despite their efficacy, calcineurin inhibitors come with challenges and potential side effects, necessitating careful monitoring and management. As research and innovation continue, the future of calcineurin inhibitors holds promise for improved therapeutic options and enhanced patient outcomes in transplantation and autoimmune disease management.