In the last decade, the field of immunotherapy of tumors has achieved remarkable results: tumor immunotherapy technologies such as PD-1/PD-L1 immunological checkpoint inhibitors and CAR-T cell therapy have been approved for marketing, humans and tumors. On the road of resistance, we will continue to move forward. So, what new tumor immunotherapy technologies will be in the future? This article will describe the most promising tumor immunotherapy technology, based on Neoantigen's immunotherapy.

It is well known that tumor cells generally have a large number of genetic mutations compared to normal somatic cells, and it is these mutations that cause uncontrolled proliferation of cancer cells. Tumor cells are often produced on the basis of gene mutations with proteins with specific amino acid sequence variations. These variant proteins are hydrolyzed by intracellular proteases and bound to MHC molecules and presented to the cell surface, which is then specific. T cells recognize and activate a cancer immune response. These tumor gene mutations cause changes in the protein sequence and are called tumor "new antigens".
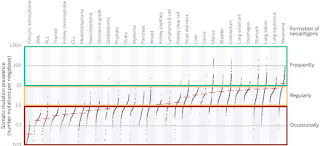
Neoantigen research
In May 2014, Science published a method to activate and screen for lymphocytes that specifically recognize cancer cells using Neoantigen technology. A patient with advanced malignant cholangiocarcinoma was successfully treated: the patient was 43 years old and was an advanced bile duct. Cancer patients, through the whole exome sequencing of the patient's tissue section, found that the patient has a total of 26 gene mutations, many of these mutations will synthesize abnormal proteins (Neoantigen). The researchers then performed all of the neoantigen's synthetic short peptides, and then co-cultured the lymphocytes isolated from the patient's tumor tissue with short peptides, and then screened out T lymphocytes that specifically recognize the ERBB2IP gene mutation and re-infused them to the patient. in vivo. In the cells that were first returned, about 25% of the cells with specific recognition ability, after the cells were returned, the patient's tumor began to shrink and stabilized for about one and a half years. After the tumor progresses again, the purity of the returned cells is increased. Of the cells returned for the second time, 95% of the cells were cells with specific abilities. After the cells were returned, the lesions in the body of the patient were found to be significantly reduced again. After a period of time, complete remission was finally achieved and the tumor completely disappeared. After lymphocyte reinfusion, it has achieved complete remission, and the effect has been maintained for several years, which is a miracle!
In 2017, the research teams from the United States and Germany independently used Neoantigen technology for cancer clinical trials, and achieved good results in Melanoma treatment. First, the researchers obtained biopsy samples from tumors in melanoma patients and sequenced them to identify cancer cell mutations. Subsequently, the researchers performed predictions of nascent antigens and vaccine synthesis based on cancer cell mutation data. Among them, the Harvard team produced 13-20 different peptide vaccines containing nascent antigens for each patient, followed by clinical trials in 6 melanoma patients with high risk of recurrence. The data show that 60% of the polypeptide causes a T cell immune response in the patient. Of the 6 patients, 4 had no signs of recurrence after two years of receiving the vaccine. Two other patients showed signs of recurrence, but achieved complete remission after receiving PD-1 immunological checkpoint inhibitor treatment. The team at the University of Mainz in Germany used another method: RNA vaccine, which produced 10 different RNA vaccines encoding new antigens for each patient and subsequently tested them in 13 melanoma patients. Sixty percent of these RNA fragments also elicit an immune response in patients. Of the 13 patients, 8 patients showed no signs of recurrence within one year after receiving the vaccine. The other 5 patients had spread of the tumor when they received the vaccine. Two of the patients had tumor shrinkage after receiving the vaccine, and the other patient also had the same Complete remission was obtained after receiving the PD-1 antibody drug.
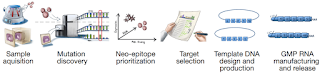
How to prepare Neoantigen
As shown in the figure below, the steps of the implementation process seem to be quite complicated. However, it is not complicated. It mainly shows the entire implementation of an individualized tumor treatment, so it looks quite complicated. It is. We only need to pay attention to the new antigen part, which is actually the leftmost column. The general process is to collect the tumor tissue first, and then simultaneously determine the DNA sequence and RNA sequence of the tumor tissue, and obtain the tumor tissue by bioinformatics analysis. Non-synonymous mutations, after which the mutant polypeptides are synthesized, and then in vitro experiments are further screened for mutant polypeptides capable of eliciting an immune response, and finally the effective peptides are determined for injection.
About us
Creative Peptides is specialized in the process development and the manufacturing of bioactive peptides. We are dedicated to offering custom peptide synthesis, process development, GMP manufacturing as well as catalog products for customers in industry and research area. Here are some our products, including: Chelate Peptides, Immunotherapy, Cell Therapy, Anti-aging peptide, etc.