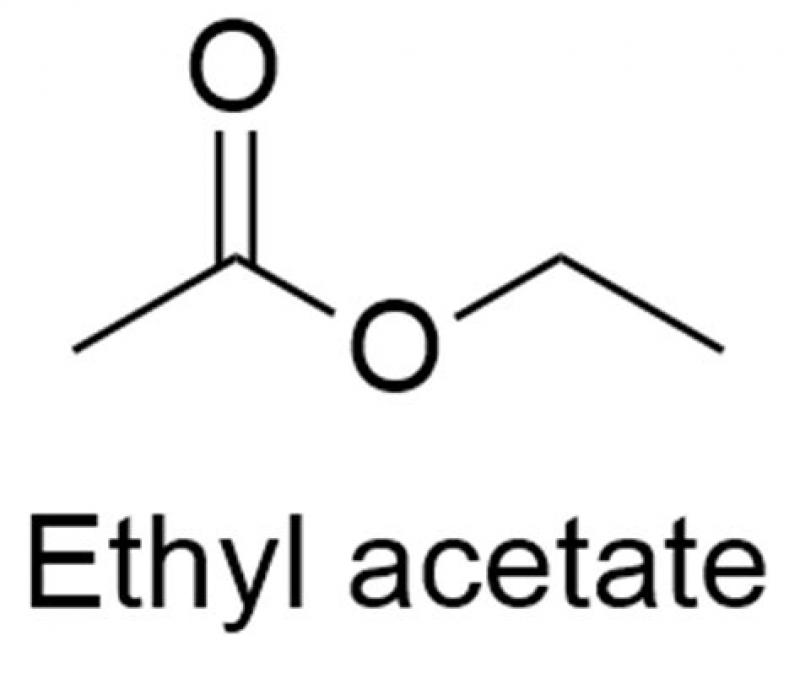
Ethyl acetate, with the chemical formula CH3COOCH2CH3, is an organic compound commonly known for its fruity, sweet smell. This volatile and colorless liquid is an ester, formed from the reaction between acetic acid and ethanol. Ethyl acetate is not only found in various fruits and flowers but also holds significant importance in both industrial and everyday applications.
Properties: Ethyl acetate exhibits several intriguing properties that make it useful in diverse fields. Firstly, its low boiling point of around 77°C (170.6°F) makes it highly volatile, allowing it to evaporate quickly at room temperature. This volatility contributes to its characteristic sweet scent, which is why it is often used in perfumes, fragrances, and flavorings. Additionally, ethyl acetate is relatively safe for human use and is classified as a low-toxicity chemical.
Uses:
-
Solvent: Ethyl acetate's most widespread use is as a solvent in various industries. It can dissolve many substances, including oils, resins, cellulose, and synthetic polymers. This property makes it invaluable in the production of paints, varnishes, adhesives, and nail polish removers.
-
Food and Beverage Industry: Ethyl acetate is used in the food and beverage industry as a flavor enhancer. Its sweet, fruity aroma makes it a popular choice for adding fragrance to confectionery, baked goods, and candies.
-
Perfumes and Fragrances: As mentioned earlier, ethyl acetate's pleasant scent finds application in the perfume and fragrance industry. It is often used as a top note in perfumes to provide a fresh and fruity aroma.
-
Extraction of Natural Products: Ethyl acetate is widely employed to extract natural compounds from various plant materials. This extraction process is utilized to obtain essential oils, flavors, and bioactive compounds from herbs and spices.
-
Nail Polish Removers: The quick evaporation and low toxicity of ethyl acetate make it a popular ingredient in nail polish removers.
-
Laboratory Applications: In laboratories, ethyl acetate serves as a solvent for chemical reactions, chromatography, and extractions. Its non-reactive nature with many compounds and relatively low cost contribute to its popularity.
-
Adhesives and Sealants: Ethyl acetate is used in the production of adhesives and sealants, where it acts as a solvent for various components and helps in the binding process.
Safety Considerations: While ethyl acetate is considered safe for many applications, it is essential to handle it with care. It is a volatile compound, so adequate ventilation is crucial when using it in enclosed spaces. Prolonged exposure to high concentrations of ethyl acetate can cause irritation to the eyes, skin, and respiratory system. Therefore, it is necessary to use protective gear such as gloves, goggles, and masks when working with this chemical.
In conclusion, ethyl acetate is a versatile compound with a wide range of applications across various industries. Its pleasant scent, low toxicity, and excellent solvent properties make it a valuable component in perfumes, flavors, paints, and many other products. However, it is essential to use this chemical responsibly and take appropriate safety precautions to ensure its safe handling.