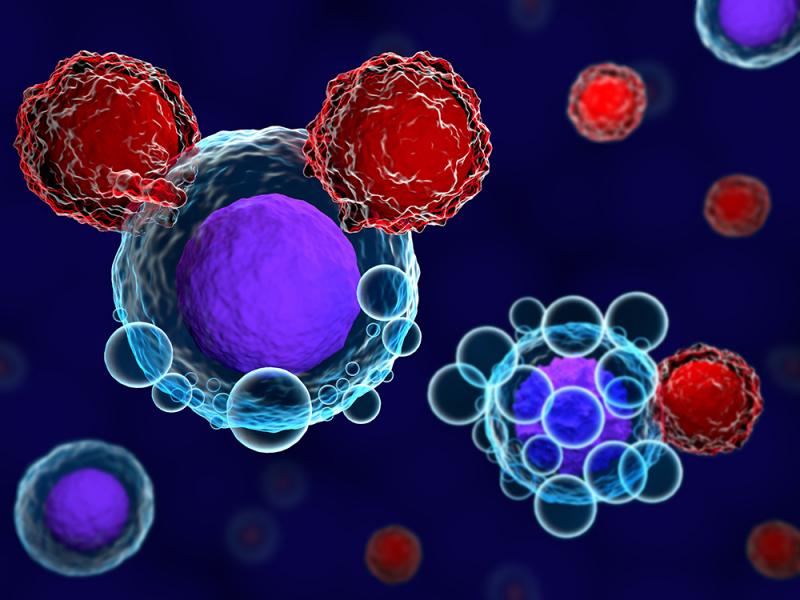
CAR T Cell Therapy, short for chimeric antigen receptor T cell therapy, represents a groundbreaking approach in the field of cancer treatment. This innovative immunotherapy harnesses the power of a patient's own immune system to combat cancer cells. With its remarkable success in treating certain types of leukemia and lymphoma, CAR T cell therapy has emerged as a game-changer, offering new hope to patients who have exhausted conventional treatment options. In this article, we will explore the concept of CAR T cell therapy, its mechanism of action, successes, challenges, and its potential for the future of cancer care.
CAR T Cell Therapy involves reprogramming a patient's T cells, a type of white blood cell, to target and eliminate cancer cells. The process begins by collecting the patient's T cells through a blood sample. These T cells are then genetically modified in a laboratory to express a chimeric antigen receptor (CAR) on their surface. The CAR is designed to recognize specific proteins, known as antigens, that are present on the surface of cancer cells.
Global CAR T Cell Therapy Market Is Estimated To Be Valued At US$ 2,259.5 Million In 2022 And Is Expected To Exhibit A CAGR Of 20.9 % During The Forecast Period (2022-2030).
The modified CAR T cells are then multiplied in the laboratory to produce a large population of these cancer-targeting immune cells. Finally, the expanded CAR T cells are infused back into the patient's body, where they seek out and destroy cancer cells that bear the targeted antigen. This personalized approach leverages the patient's own immune system, empowering it to recognize and eliminate cancer cells more effectively.
CAR T Cell Therapy has shown remarkable success in the treatment of certain hematological malignancies, particularly acute lymphoblastic leukemia (ALL) and certain types of non-Hodgkin lymphoma (NHL). In clinical trials and real-world applications, CAR T cell therapy has achieved impressive response rates, including complete remission, in patients who have not responded to conventional therapies or have relapsed after prior treatments. The results have been particularly promising in pediatric and young adult patients with ALL, where conventional treatments have limited effectiveness.
The success of CAR T Cell Therapy lies in its unique mechanism of action. Unlike traditional chemotherapy or radiation, which target both healthy and cancerous cells, CAR T Cell Therapy is highly targeted and selective. The genetically modified CAR T cells are engineered to specifically recognize and bind to cancer cells, sparing healthy cells from damage. This targeted approach not only enhances the efficacy of treatment but also minimizes side effects commonly associated with traditional cancer therapies.
One of the most remarkable success stories of CAR T Cell Therapy is the treatment of pediatric and young adult patients with relapsed or refractory ALL. In clinical trials, CAR T cell therapy has demonstrated response rates exceeding 80%, with a significant number of patients achieving complete remission. These results have led to the approval of CAR T cell therapies, such as Kymriah® and Yescarta®, by regulatory authorities in several countries, offering new treatment options for patients who have limited alternatives.