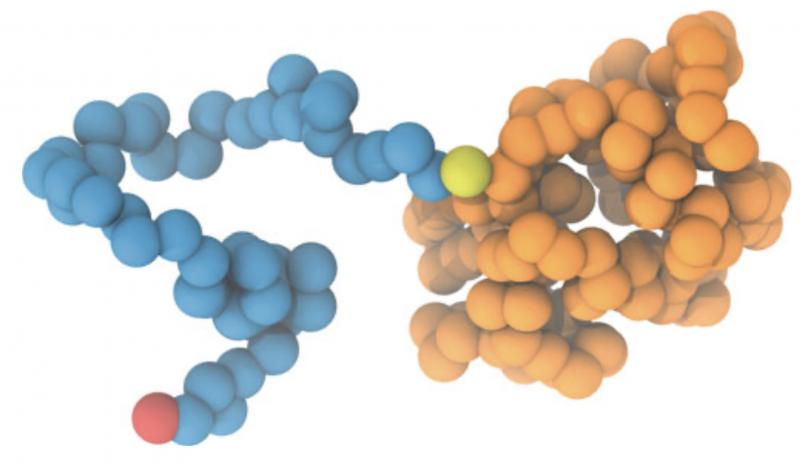
PEGylation is a revolutionary technique that involves covalently attaching polyethylene glycol (PEG) chains to proteins, enhancing their pharmacokinetic properties and therapeutic efficacy. PEGylation has transformed the landscape of biopharmaceuticals, allowing for improved drug delivery, extended half-lives, reduced immunogenicity, and enhanced stability of therapeutic proteins. In this comprehensive article, we explore the science behind PEGylated proteins, their applications in medicine, and their impact on patient care and treatment outcomes.
The Science of PEGylation
PEGylation is achieved by attaching PEG molecules to specific amino acid residues on the surface of proteins. The attachment is typically through a chemical bond, creating a covalent linkage between the PEG chains and the protein. The hydrophilic nature of PEG creates a protective shield around the protein, reducing its interaction with the body's immune system and preventing rapid clearance by the kidneys or liver.
Advantages of PEGylation
-
Extended Circulation Half-Life: PEGylation significantly prolongs the circulation half-life of proteins by reducing their recognition and clearance by the reticuloendothelial system. This extension allows for less frequent dosing, improving patient compliance and treatment convenience.
-
Improved Stability: PEGylation provides a protective barrier around the protein, shielding it from degradation and denaturation. This increased stability enhances the shelf life of PEGylated therapeutic proteins and reduces the need for strict temperature controls during storage and transport.
-
Reduced Immunogenicity: PEGylation reduces the immunogenicity of therapeutic proteins, minimizing the risk of an immune response or the development of neutralizing antibodies. This is particularly important for chronic treatments where long-term efficacy is crucial.
-
Enhanced Solubility: PEGylation often improves the solubility of proteins, facilitating their formulation into injectable dosage forms and enhancing their bioavailability.
-
Improved Tissue Penetration: The hydrophilic PEG chains enhance tissue penetration of PEGylated proteins, potentially allowing for better distribution and targeting of drugs to specific sites of action.
Applications in Medicine
PEGylation has found extensive applications in various fields of medicine, particularly in the development of biopharmaceuticals and targeted therapies. Some of the key areas where PEGylated proteins have made a significant impact include:
-
Oncology: PEGylated proteins are extensively used in cancer treatment, where they improve the pharmacokinetics and efficacy of chemotherapeutic agents. PEGylation reduces toxicity to healthy tissues, increases drug half-life, and enhances tumor accumulation, leading to more effective and tolerable cancer therapies.
-
Hemophilia Treatment: PEGylated recombinant factor VIII and factor IX are utilized to treat hemophilia, extending their circulatory half-lives and reducing the frequency of injections required for effective bleeding control.
-
Autoimmune Disorders: PEGylated proteins have shown promise in the treatment of autoimmune disorders, such as rheumatoid arthritis and multiple sclerosis, by reducing disease activity and slowing down disease progression.
-
Enzyme Replacement Therapies: PEGylation improves the stability and half-life of therapeutic enzymes used in enzyme replacement therapies for genetic disorders such as Gaucher's disease and Fabry disease.
Regulatory Considerations
The development and approval of PEGylated proteins involve specific regulatory considerations. Regulatory agencies, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), evaluate the safety, efficacy, and immunogenicity of PEGylated products through rigorous preclinical and clinical studies. Special attention is paid to potential PEG-related adverse effects, such as hypersensitivity reactions or PEG-specific antibodies.
Challenges and Future Directions
Despite the numerous advantages, PEGylation also presents some challenges. The PEGylation process may alter the biological activity or binding affinity of the protein, necessitating careful optimization during drug development. Additionally, the potential formation of anti-PEG antibodies in some patients requires ongoing monitoring to assess their impact on safety and efficacy.
Researchers and scientists continue to explore innovative strategies to further optimize PEGylation, such as site-specific PEGylation, where PEG chains are attached at predefined sites on the protein to minimize interference with its function. Furthermore, advancements in PEGylation technology are paving the way for novel therapeutic modalities, including PEGylated RNA-based therapies and PEGylated nanoparticles for drug delivery.
Conclusion
PEGylated proteins have revolutionized the landscape of biopharmaceuticals, providing enhanced therapeutic options for patients across various medical fields. By extending circulation half-lives, reducing immunogenicity, and improving stability, PEGylation has significantly improved the pharmacokinetic properties and clinical efficacy of therapeutic proteins. As research and technology continue to progress, the future of PEGylation holds even greater promise, fostering the development of more effective and targeted treatments to improve patient care and outcomes in the realm of precision medicine.